Nested Applications
MAL26 - Guidelines for abstract submission
EFORT CONGRESS MÁLAGA 2026 | GUIDELINES FOR ABSTRACT SUBMISSION
Abstract Submission for the EFORT congress 2026 is NOW CLOSED! All abstracts received for the EFORT Congress Málaga 2026 Scientific Programme will be under review by our EFORT Science Committee appointed topic experts through our abstract submission and management online platform: https://scientific.efort.org/efort2026.
Abstracts must correspond to previously unpublished work. EFORT reserves the right to reject your abstract if previously presented. EFORT has focus on ethical research misconduct. Any kind of plagiarism or fraud like fabrication and falsification of data will not be tolerated and handed over to the corresponding responsible authorities.
In order to get your abstract selected for presentation at an EFORT meeting it should convey information that blinded scorers can use to identify good quality research. It must fit within stated parameters for length and layout. Abstracts not complying with these requirements will be rejected.
The abstract submission system is self-explanatory and requires that a number of mandatory fields are completed, which makes it very difficult to submit in an incorrect format. You may wish to consider writing your abstract in appropriate sections before beginning your submission, but do not worry – it is easy to draft and change your abstract online before finally submitting.
EFORT aims to showcase the best available research to the widest audience of trauma and Orthopaedic Surgeons in Europe.
MAL25 Abstract submission guidelines Tabs
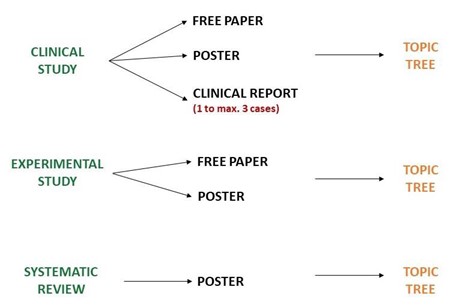
Submission is defined by an initial branching to differentiate Clinical Vs. Basic Science Research as well as Review abstracts. An initial choice to submit your abstract either as a Clinical Study, as an Experimental Study or as a Systematic Review is essential to allow the good assignment of the work. Please choose carefully your main category:
- Clinical Study: presentation is possible as Free Paper or Poster (clinical reserach with control group), or as Clinical Report (1 to max. 3 specific clinical cases).
- Experimental Study: presentation is possible as Free Paper or Poster.
- Systematic Review: only Poster presentation is possible.
Abstracts should be in English. Diagrams, tables and pictures should not appear in abstracts, only text should be provided. The following structure is mandatory:
- Title: minimum 25 / maximum 250 characters.
- Keywords: minimum 3 / maximum 7 keywords – chose these so that people can identify your abstract on an electronic search using these words as search terms.
- Abstract: minimum 1000 / maximum 3600 characters – number of characters per section are not restricted. All sections are mandatory.
The content is provided in 5 different text boxes corresponding to the sections building the core abstract. You should aim to split your content according to each box, or you may miss out on scoring opportunities during the selection process. There is no need to retype the headings in the text box and copy-paste options from other text files can be used.
Abstracts submitted as Free Papers / Posters
- Background – Why is your study needed? What do we know already and what gaps are there?
- Objectives – What is the research question your study aims to answer?
- Design and Methods – Outline how the study was conducted.
- Results – Briefly summarise the findings, quoting supporting data.
- Conclusion – Briefly discuss your results and tell the scorers what your study adds – only base conclusions on your own findings.
Abstracts submitted as Clinical Report
- Background – Introduce the condition and indicate what we know already
- Case Presentation – Describe relevant history, examination findings, test results and interventions
- Outcomes – What happened and could you measure it? What was the end result?
- Discussion – Describe how this case adds to our knowledge, what questions it raises and whether it should affect management of similar cases.
Authors Instructions
A maximum of 12 co-authors should be included in the exact order to appear in the authorship list. Several fields are mandatory for each author. Before starting the submission process, please make sure you do have the necessary information as follows:
- First Name
- Last Name
- Complete affiliation (including institution, city and country)ä
- Corresponding email address
- Medical qualification (from below options)
- Orthopaedic Surgeon (> 3 years experience)
- Orthopaedic Surgeon (< 3 years experience)
- Resident
- Trainee
- Medical Student
- Other in Medical industry
- None - Profession (from the below options)
- Orthopaedic Physician
- Non-orthopaedic Physician
- Hospital Administrator
- Physiotherapist
- Nurse
- Medical industry
- Journalist
- Other - Conflict of Interest declaration (each question to be answered separately with No/Yes*):
* the Company Name will be requested
1. The author has held shares in a commercial party related directly or indirectly to the subject of this abstract in the past three years.
2. The author has received royalties from a commercial party related directly or indirectly to the subject of this abstract in the past three years.
3. The author has done consulting work for a commercial party related directly or indirectly to the subject of this abstract in the past three years.
4. The author has given paid presentations for a commercial party related directly or indirectly to the subject of this abstract in the past three years.
5. The author has received institutional support from a commercial party related directly or indirectly to the subject of this abstract in the past three years.
6. The author has attended meetings with the sponsorship of a commercial party related directly or indirectly to the subject of this abstract in the past three years.
- Reviewers can only score on the information contained in the abstract.
- It must be an honest representation of work you have completed with no estimates or projections based on data available at the time of submission.
- The final presentation must reflect the data contained in the abstract and any manipulation after submission will be considered fraudulent.
- Do not include the name of your institution or any of the researchers in the content of the abstract (title and/or inside the 5 core sections).
- Avoid any city/country allusion.
- Do not use abbreviations which have not been specified first.
- The abstracts will be scored against objective criteria by orthopaedic surgeons of the relevant subspecialty and in a blinded fashion.
- Each abstract is scored by a number of independent observers and the mean score is used in determining the cut-off point for acceptance.
- Abstracts must score highly across the full range of parameters in order to be selected so please pay full attention to each section of the abstract when compiling it.